Simple Tips About How To Tell If A Molecule Has Dipole Moment
Organic chemistry (morsch et al.) 2:
How to tell if a molecule has a dipole moment. Chemistry (zumdahl and decoste) 13: This organic chemistry video tutorial provides a. A wide difference in the electronegativity of the bonded atoms causes polarity in the bond, and the bond has.
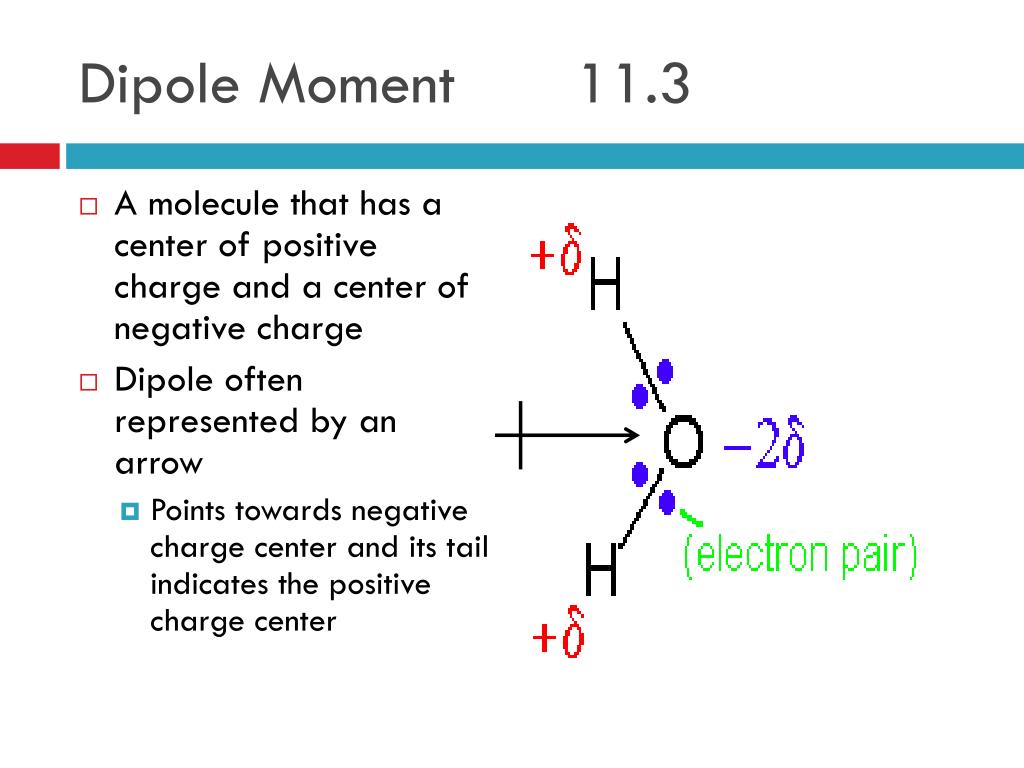
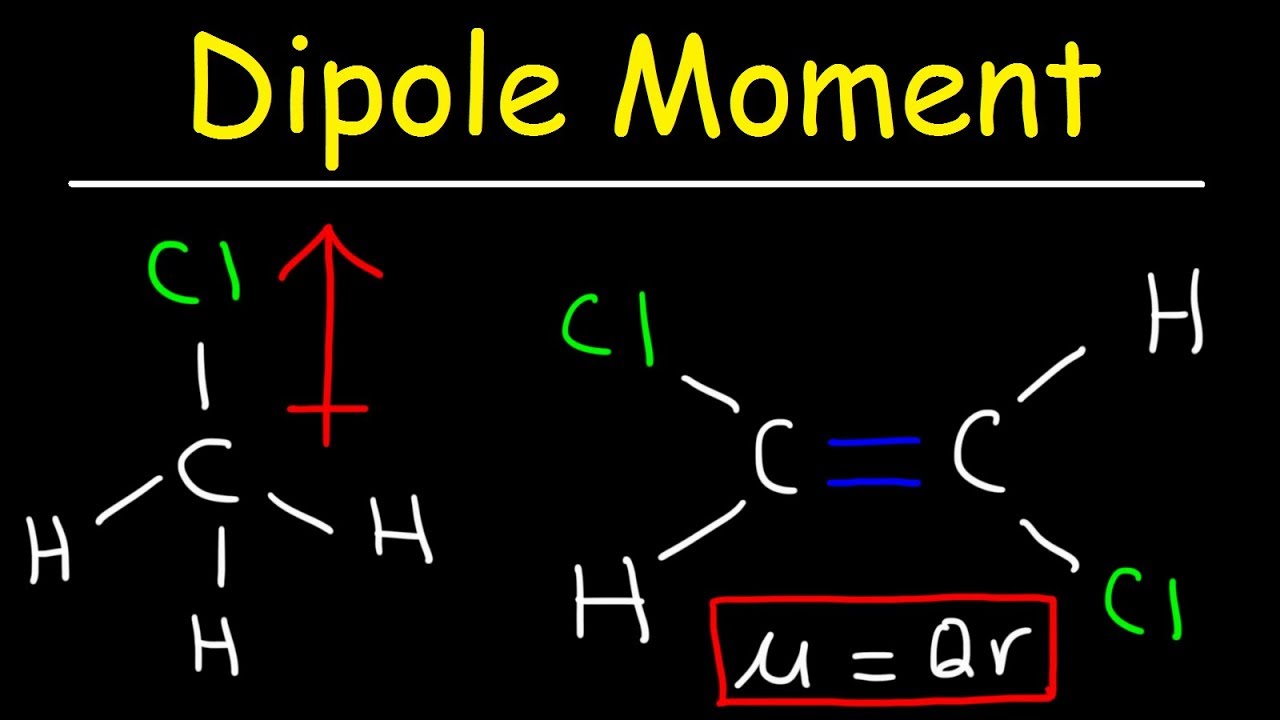
It is denoted by ‘d’. Dipole moments tell us about the charge separation in a molecule. The molecule possesses a dipole moment, μ, which is equal to the magnitude of the charge, e, multiplied by the distance, d, between the centers of charge:
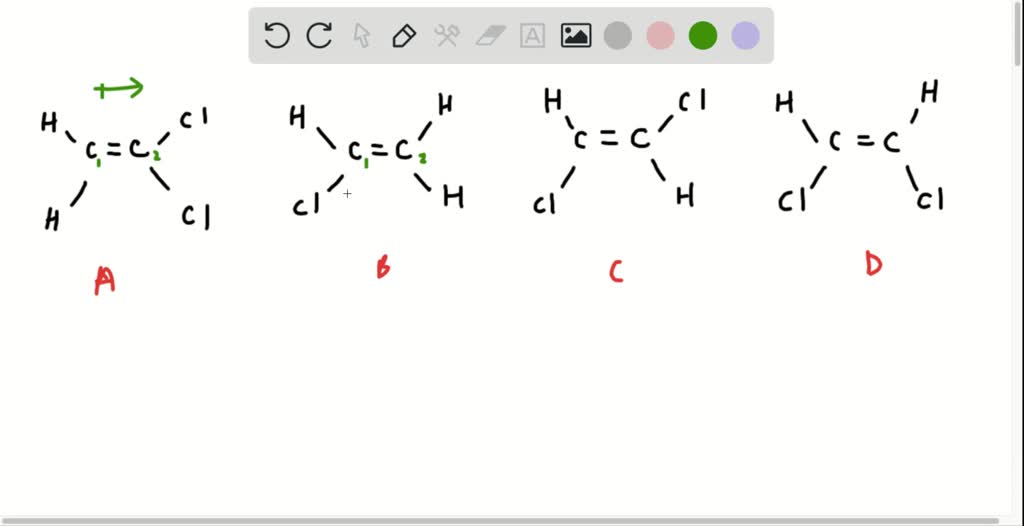
Determine if the molecule is polar or not by showing the corresponding dipole moment (s). 143k views 9 years ago. 601k views 5 years ago new organic chemistry playlist.
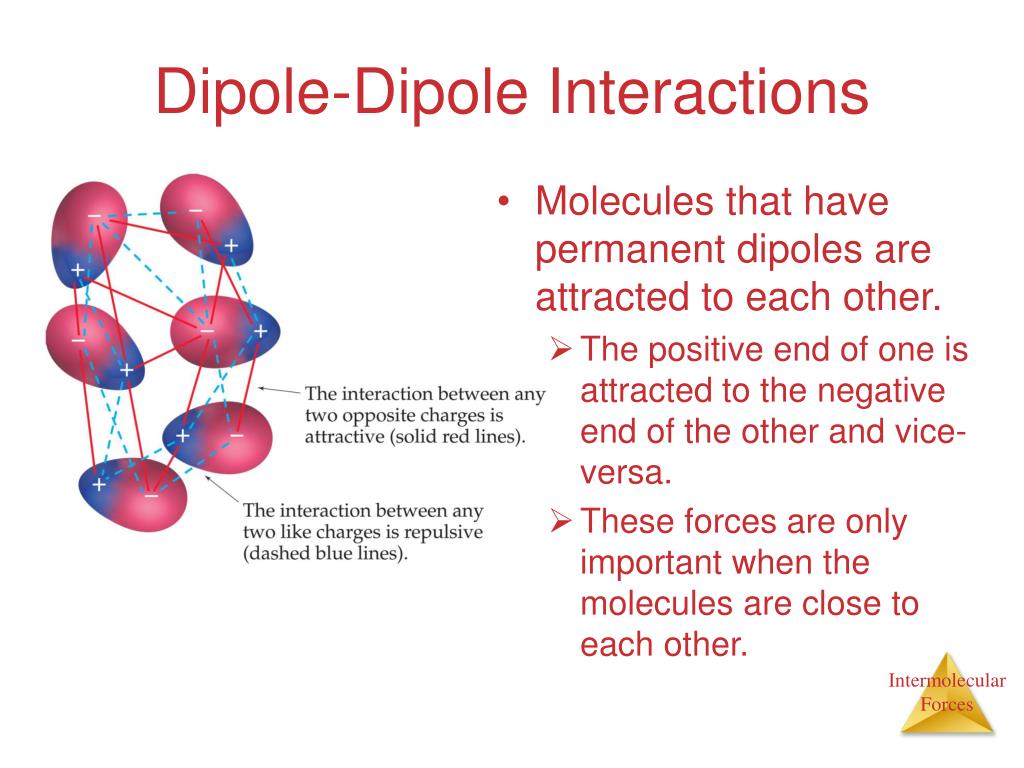
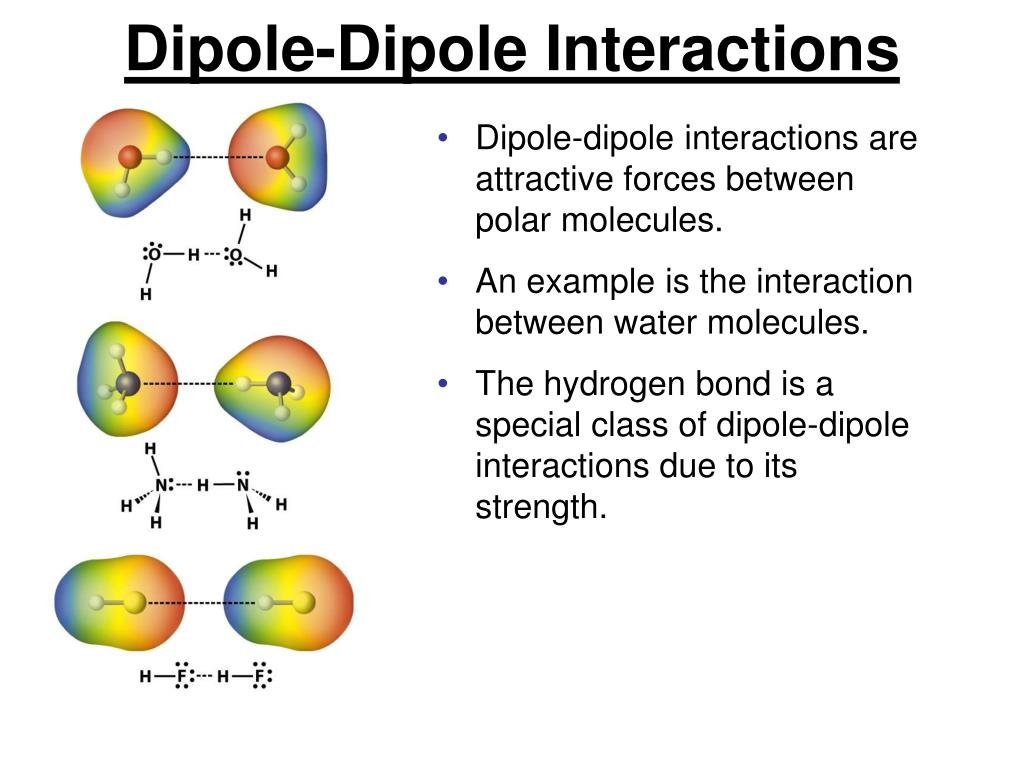
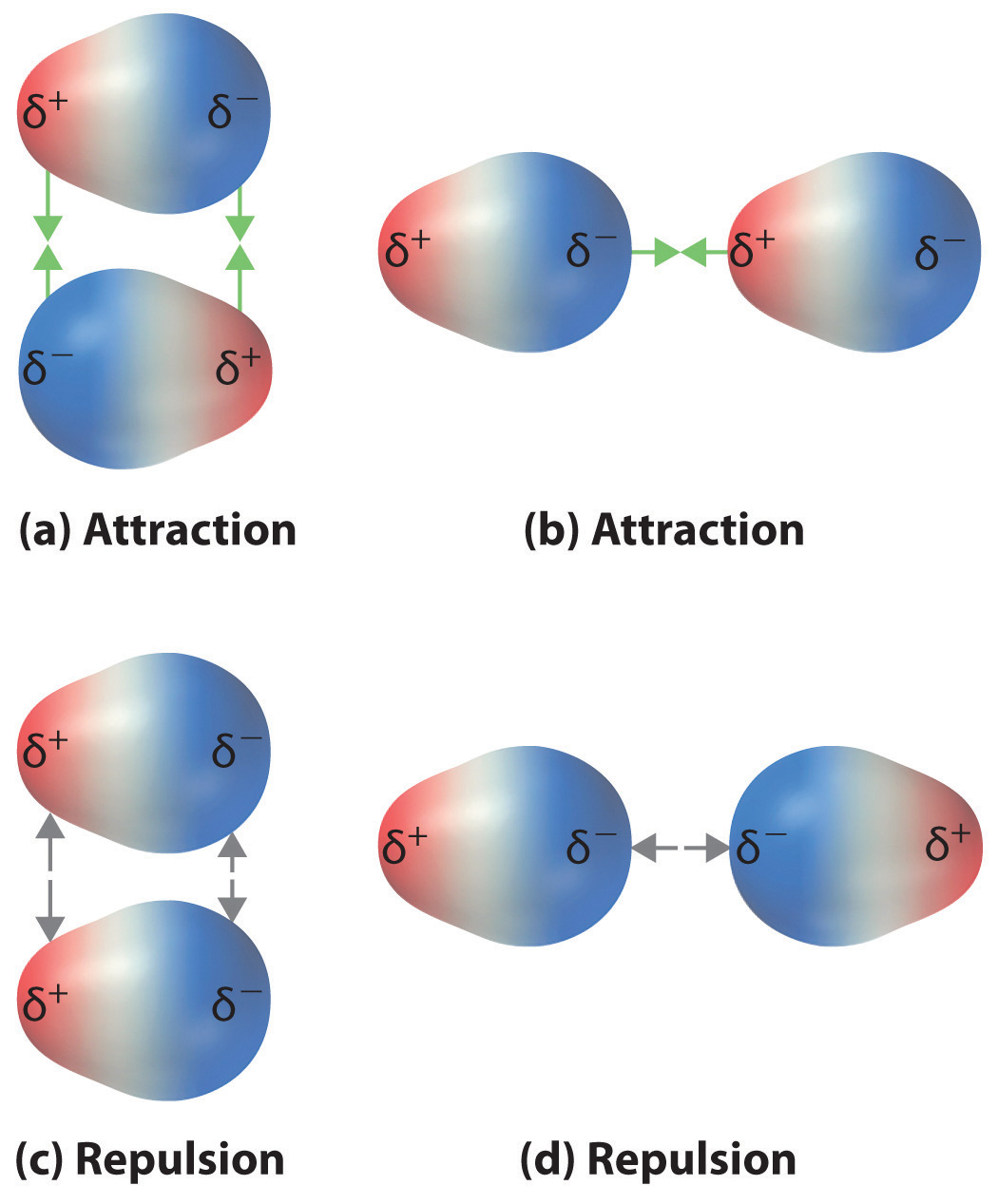
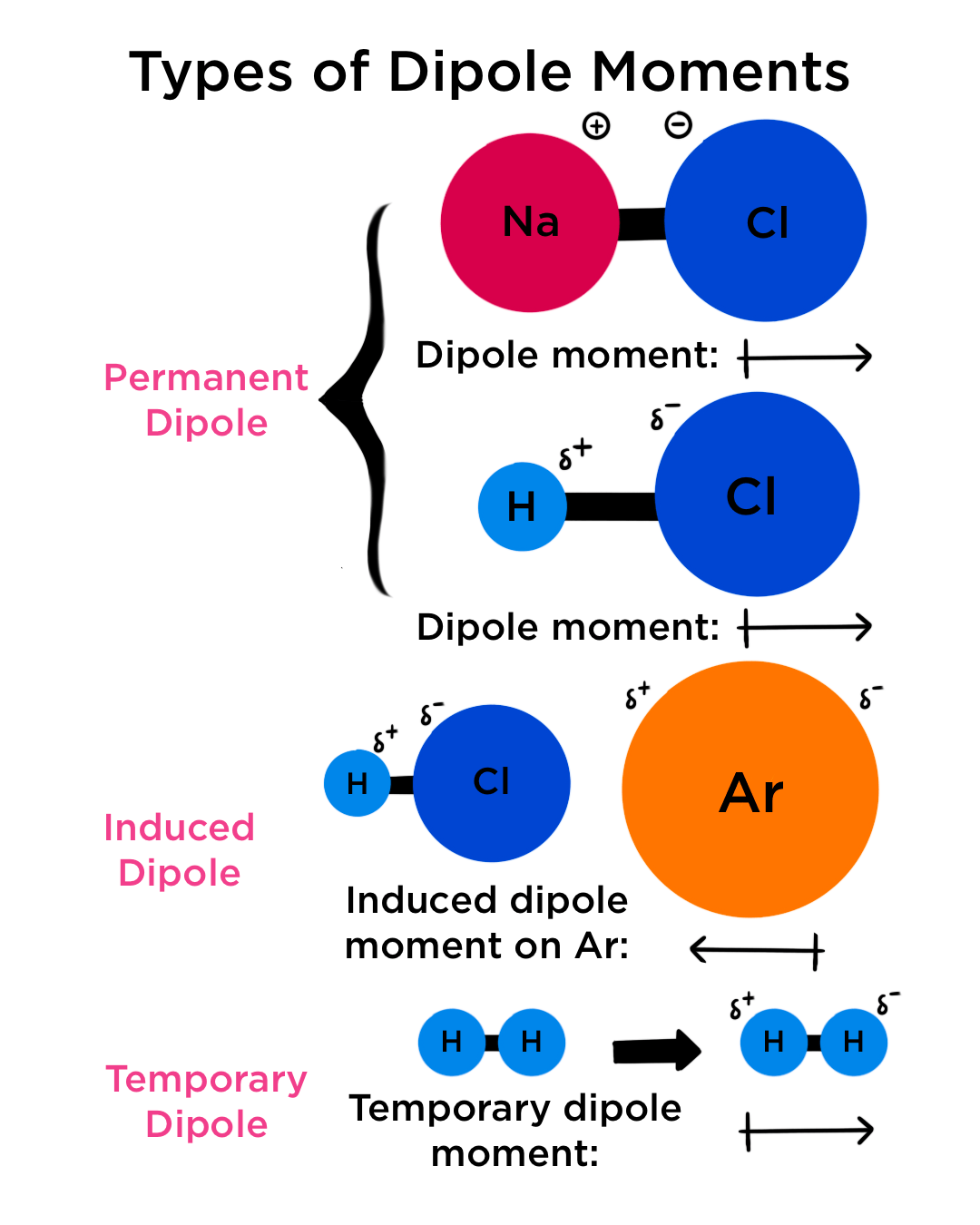
Dipole moments occur when there is a separation of charge. Dipole moments arise from differences in electronegativity. A molecular dipole is the sum of all the.
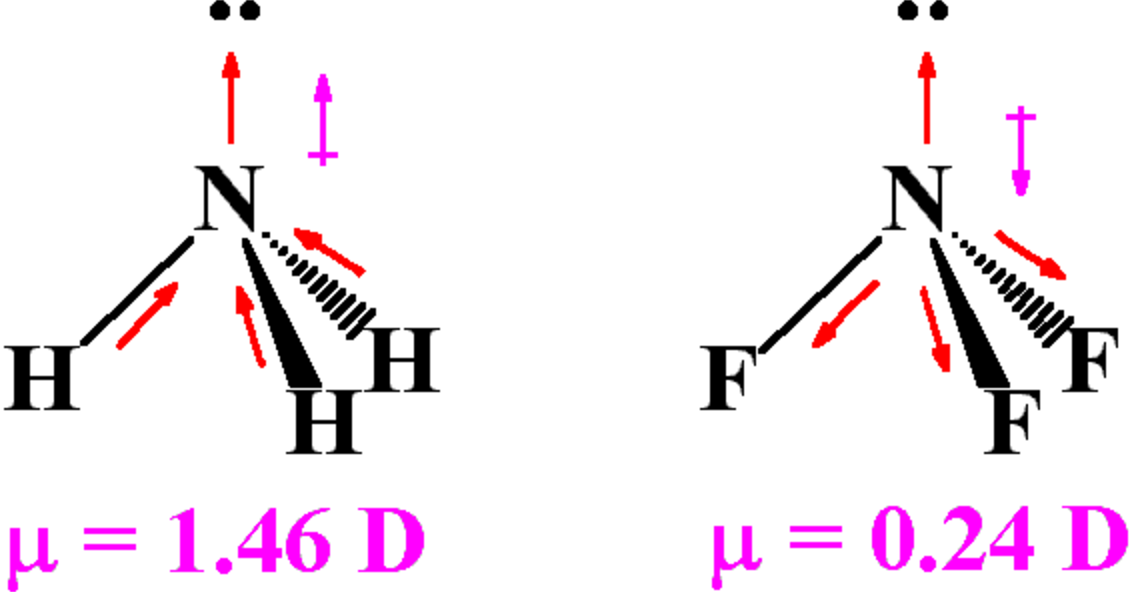
How to determine whether a molecule has an overall molecular dipole moment. The dipole moment (μ) is the calculation of the net molecular polarity at either end of the molecular dipole, which is the magnitude of the charge q times the distance r between. We indicate a bond dipole by an arrow with a + at one end and pointing towards the negative end of the bond.
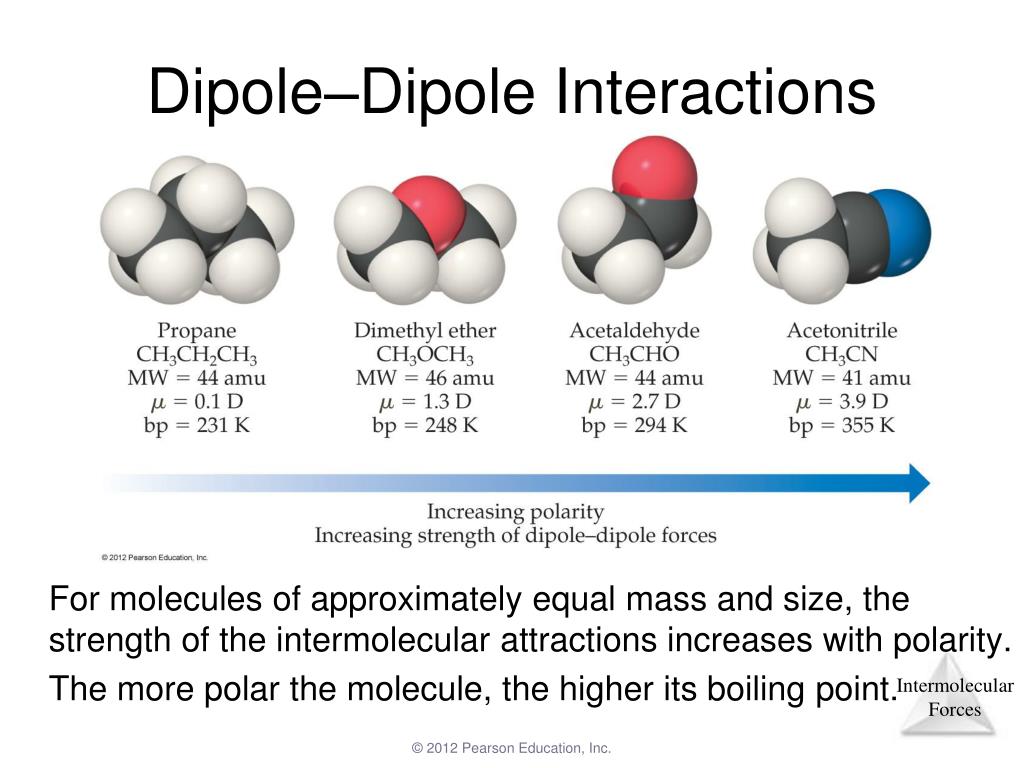
For molecules of similar size and mass, the strength of these. 1d = 3.33564 × 106−30c. If δen < 0.5, we usually say that the bond is.
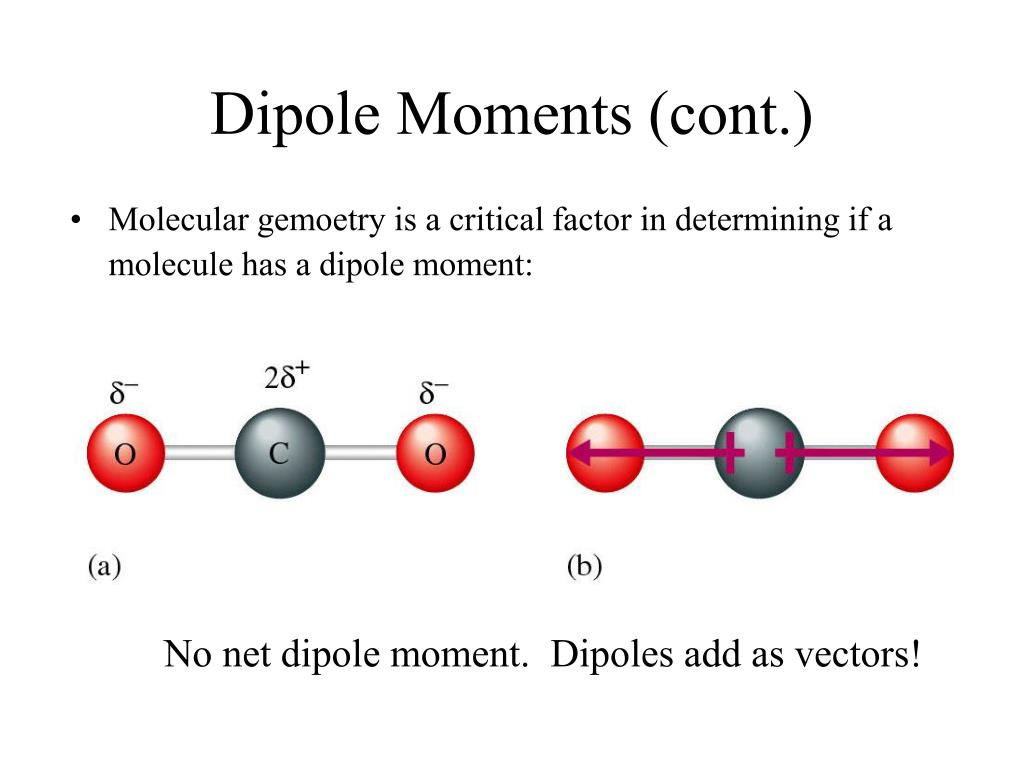
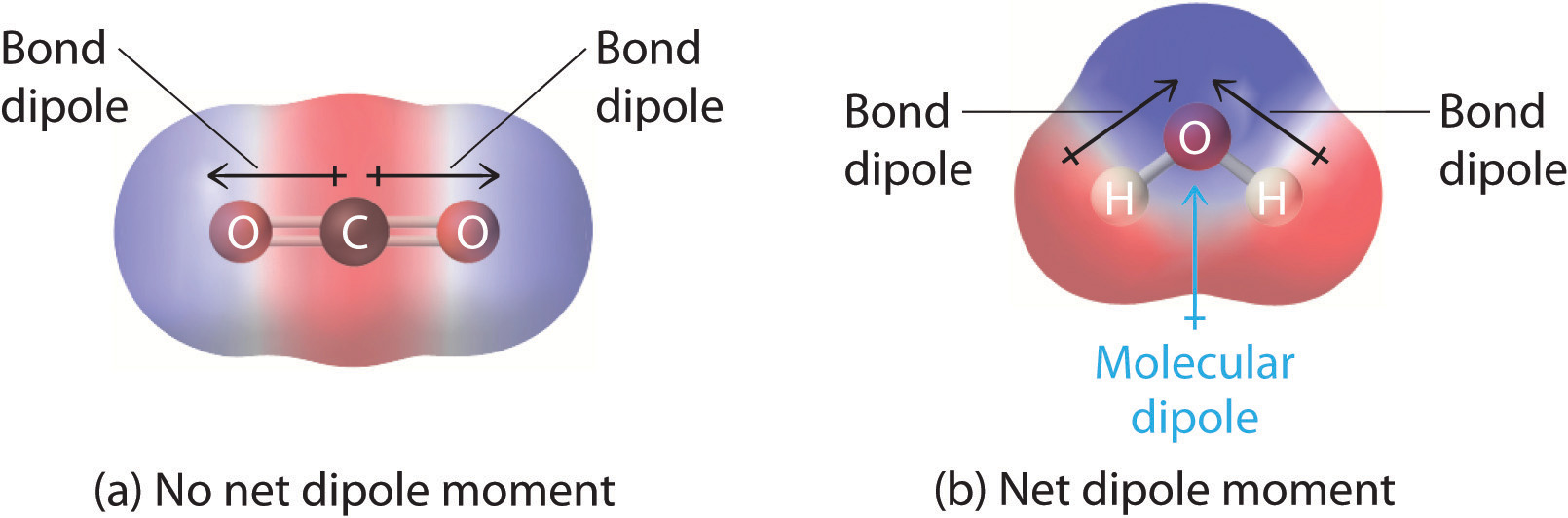
Predict if a molecule has a dipole moment. Mathematically, dipole moments are vectors, and they possess both a magnitude and a direction. These charges are equal in magnitude but.
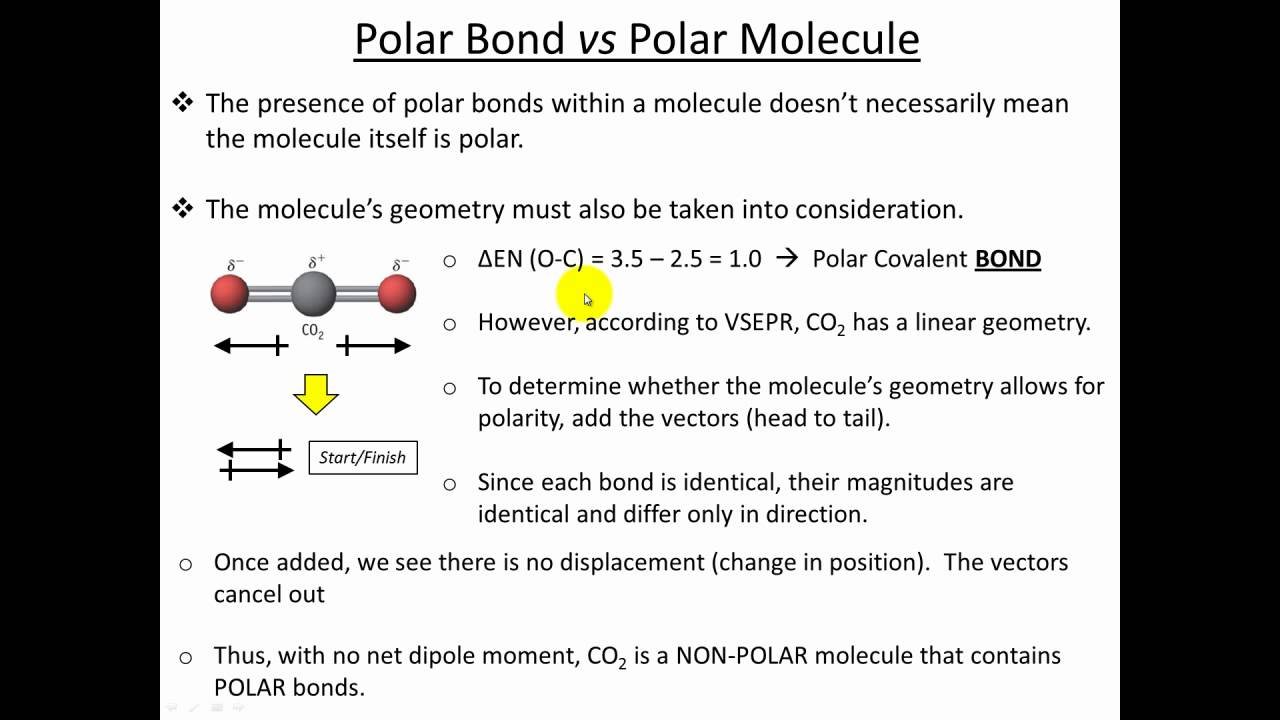
Molecular polarity depends on both individual bond polarities and molecular geometry, the latter of which we can predict using vsepr theory. This molecule is formaldehyde and has a trigonal planar geometry according to the. Using the cross bow arrow shown below we can show that it has a net dipole.
Mathematically, dipolemoment(μ) = charge(q) × distanceofseparation(r) the dipole moment is measured in debye units. We indicate the dipole moment by writing an arrow above the molecule. Bond polarity and dipole moments.
They can occur between two ions in an ionic bond or between atoms in a covalent bond; The net dipole is the measurable, which is called the dipole moment. The larger the difference in.